Corona: Next vaccine study starts
Victoria Becker completed a Bachelor of Arts in “Online Editing” at the TH Köln and wrote a practical thesis at the Goethe-Institut Lithuania. She is currently studying media and communication sciences as a master at the University of Lund, Sweden, and writes for, among other things.
More about the experts All content is checked by medical journalists.Another German corona vaccine is allowed to enter the first phase of clinical testing: The vector vaccine was developed with the cooperation of German universities and is based on a well-known smallpox vaccine. How is it tested?
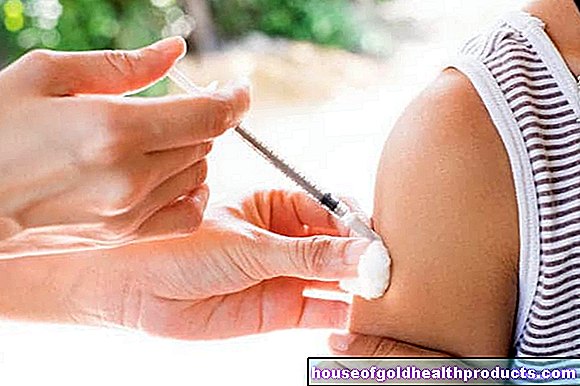
Another corona vaccine has been approved for clinical testing on test subjects in Germany. It is a so-called vector vaccine, with which genetic material of the Covid-19 pathogen is smuggled into the body.
It was developed by the German Center for Infection Research (DZIF) and IDT Biologika GmbH in Dessau. The Ludwig Maximilians University of Munich, the Philipps University of Marburg and the University Medical Center Hamburg-Eppendorf are also involved. The Paul Ehrlich Institute (PEI), which is responsible for vaccines, approved the study on September 30th.
How does the vaccine work?
The vaccine is a so-called vector vaccine. In this type of vaccine, genetic material from the pathogen against which the vaccination is intended to act is inserted into a harmless carrier virus and then injected as a vaccine. A weakened vaccine virus, for example, serves as the carrier virus, also known as a vector. This is how the genetic information, in this case that of the coronavirus, reaches the cells of the vaccinated person.
For the production of the vaccine, which has now been approved for testing, the genetic information of a Sars-CoV-2 surface protein was incorporated into a modified smallpox virus. The "basic virus" has been around for more than 30 years. It was used, among other things, for the development of a vaccine against MERS (Middle East Respiratory Syndrome or Middle East Respiratory Syndrome).
The vector, i.e. the carrier virus, cannot multiply. The genetic material that has been smuggled in can simulate an infection and trigger the production of antibodies and T cells. T cells are a subset of the white blood cells that make up part of the immune system. You can find out more about the structure and function of the immune system on our overview page.
Clinical phase: how does the test work?
A vaccine has to go through a number of phases before it can be approved. In the first phase of the clinical trial, the vaccine is tested for safety, tolerability and its specific immune response against the pathogen. A quick overview of the upcoming test:
- Test participants: 30 healthy adults between 18 and 55 years of age
- Two vaccinations four weeks apart
- Measurement of antibody and T-cell formation in the body by working groups in Hamburg and Marburg
- Comparison of results with the immune response of recovered Covid-19 patients
Before the vaccine can be submitted for approval, the vaccine has to withstand two further phases of clinical testing.
German developments "can keep up worldwide"
According to the World Health Organization (WHO), 41 Covid-19 vaccine candidates are currently in clinical trials worldwide. Some of them are already in the third phase, which is important for approval. In Germany there are now six vaccines in various phases of clinical testing. According to PEI, there are two vector vaccines and four so-called RNA vaccines.
Vector and RNA vaccines differ mainly in how the genetic information of the coronavirus gets into the human body: The genetic material in RNA vaccines does not require a carrier virus, i.e. no vector, but is transported by liquid fat droplets (nanoparticles).
PEI President Klaus Cichutek said the DZIF's successful application was proof "that academic developments from Germany can also keep up worldwide". He expects that more vaccine candidates will be admitted to the test in the next few months. (dpa / vb)