Coronavirus vaccine AstraZeneca (Vaxzevria)
Updated onMaximilian Reindl studied chemistry and biochemistry at the LMU in Munich and has been a member of the editorial team since December 2020. He will familiarize himself with medical, scientific and health policy topics for you in order to make them understandable and comprehensible.
More posts by Maximilian Reindl All content is checked by medical journalists.The Covid-19 vaccine from the manufacturer AstraZeneca called Vaxzevria (AZD1222) belongs to the class of vector vaccines. It has had provisional European market approval since January 29, 2021. Read here what you know about efficacy, tolerability and application.

STIKO recommends a second vaccination with mRNA vaccines
In a communication from the Robert Koch Institute dated July 1, 2021, the Standing Vaccination Commission (STIKO) proposes a change in its recommendations for the vector vaccine Vaxzevria.
The STIKO suggests that a so-called heterologous vaccination scheme should be carried out from now on. This means that people of all ages who have already received a first dose of the AstraZeneca preparation should be vaccinated a second time with an mRNA vaccine in the future.
According to the RKI notification of July 1, 2021, this combined administration of two different coronavirus vaccines should be clearly superior to a homologous vaccination scheme. In its draft resolution, the STIKO specifies the following vaccination intervals:
- Second vaccination with Comirnaty (BioNTech / Pfizer): 3 to 6 weeks
- Second vaccination with Moderna: 4 to 6 weeks
For more information on the current study situation regarding the combined vaccination of vector and mRNA vaccines, read here.
In the current phase of the vaccination campaign, doctors are administering the Vaxzevria vaccine (AZD1222) in a homologous series of vaccinations - consisting of two doses of Vaxzevria. In order to build up the best possible vaccination protection, they are ideally administered every three months (around 84 days).
A single vaccination dose of VaxZevria corresponds to an amount of 0.5 milliliter, which is preferably injected into the upper arm.
What kind of vaccine is it?
The vaccine Vaxzevria (AZD1222) from the manufacturer AstraZeneca is the first approved vector vaccine against the disease Covid-19 in the European Union. It trains the human immune system against the Sars-CoV-2 pathogen. In clinical studies, Vaxzevria (AZD1222) provided good protection against Covid-19.
Vector vaccines belong to the class of genetic vaccines. The basis in this case is a harmless cold virus (adenovirus) from chimpanzees, which serves as a so-called vector. The vaccine virus is equipped with a short section of the Sars-CoV-2 genome, which contains the blueprint for the production of the spike protein.
With the vaccination, the blueprint gets into the human cell. This then begins to produce the virus protein: Then it presents it on its surface. The human immune system then specifically forms antibodies and immune cells (T cells, B cells) against the spike protein. This learned immune response can protect the vaccinated person from the outbreak of Covid-19 in the event of an infection.
Vaxzevria (AZD1222) has a conditional marketing authorization from the European Medicines Agency (EMA) for the European market. This means that the approval of Vaxzevria (AZD1222) is linked to requirements with regard to safety and effectiveness. These requirements are continuously and closely monitored and checked by experts from the Paul Ehrlich Institute (PEI) and the EMA.
You can find out more about how vector vaccines work in our article on vector vaccines.
Effectiveness against Covid-19
According to the RKI, the AstraZeneca vaccine has an effectiveness of 80 percent. The protection against severe courses is almost 100 percent, especially for seniors.
Vaxzevria (AZD1222) is fully vaccinated two weeks after the second vaccination dose.
Efficacy data in children and adolescents up to 18 years of age are not available. The vaccine Vaxzevria (AZD1222) is therefore not approved for this age group in the European Union.
Tolerability and side effects
The AstraZeneca vaccine is generally well tolerated. Occurring side effects will continue to be closely monitored and continuously updated by the Paul Ehrlich Institute (PEI). Serious side effects associated with the AstraZeneca vaccination are still very rare.
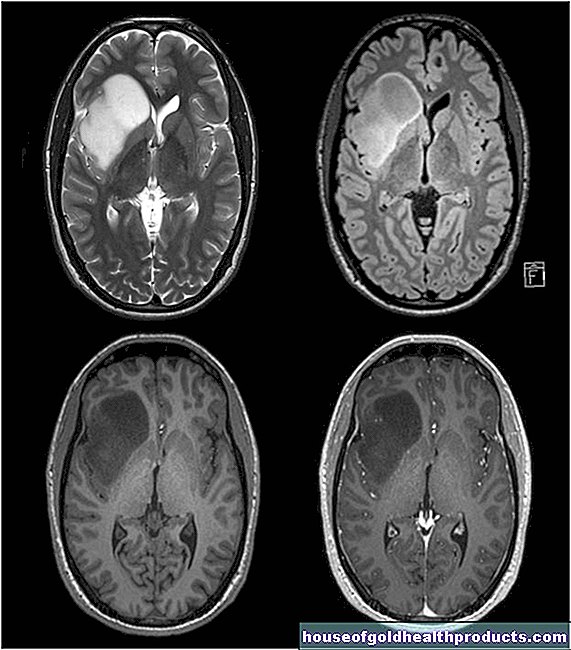
However, there were increasing reports of very rare cerebral vein thrombosis (sinus vein thrombosis) associated with a lack of blood platelets. By the end of March, 31 cases (29 women, 2 men) had been reported to the German Paul Ehrlich Institute, nine were fatal. The probability of this vaccination complication is 1 in 100,000.
The PEI emphasizes that all people who feel increasingly unwell after a vaccination with Vaxzevria (AZD1222), develop punctiform skin bleeding or severe persistent headache, should seek medical treatment immediately.
Since the symptoms of cerebral vein thrombosis did not appear immediately after vaccination, but after four to 16 days, headaches that typically appear immediately after vaccination are usually not a cause for concern.
In the meantime, Germany and other European countries recommend vaccination only for the elderly until further notice - in Germany for people over the age of 60.
The Standing Vaccination Commission (STIKO) adjusted its vaccination recommendation on May 12, 2021 for younger people under the age of 60: People who have already received an initial vaccination with the AstraZeneca vaccine should receive an mRNA vaccine (Comirnaty , Moderna) (heterologous vaccination schedule).
Further information on the combined vaccination of the AstraZeneca vaccine and the BioNTech vaccine is available here.
frequent side effects
However, around one in ten people who are vaccinated develop moderate side effects in response to the vaccination. They correspond to those that usually occur after vaccinations. The side effects usually go away within a few hours or days and include:
- mild to moderate pain or swelling at the injection site
- headache
- Exhaustion
- Joint pain
- mild feeling of illness
- Shivering
- light fever
Serious side effects
Serious side effects, such as severe (anaphylactic) reactions, are very rare after vaccination.
Cerebral vein thrombosis
However, various cases of rare, dangerous cerebral vein thrombosis have been observed, often in connection with a lack of blood platelets (thrombocytopenia). Some of the patients died from it. According to the data, around one in 100,000 people vaccinated is affected. The cerebral vein thrombosis occurred four to 16 days after vaccination and mostly affected young and middle-aged women. AstraZeneca is currently only vaccinated in people aged 60 and over.
Corresponding warnings will be promptly included in the technical information and instructions for use.
When examining blood samples from affected patients, researchers from the University Medical Center Greifswald apparently found a possible cause for the observed incidents. Accordingly, in rare cases, platelets are activated by the vaccination - similar to the processes involved in wound healing. This could be a possible explanation for the events observed. However, there is still no reliable data on this.
The PEI emphasizes that all people who feel increasingly unwell after a vaccination with Vaxzevria (AZD1222), develop punctiform skin bleeding or severe persistent headache, should seek medical treatment immediately.
Capillary leak syndrome
In addition, the manufacturer AstraZeneca recently reported on very rare cases of the so-called capillary leak syndrome (CLS), which occurred in connection with a Vaxzevria vaccination. One case with a fatal outcome was named.
According to initial estimates, this severe form of side effect affects one case in around five million vaccine doses. According to the PEI, however, in some cases those affected already had a history of CLS. So there is now a contraindication for all vaccinated people who have previously survived a CLS episode. You must no longer be vaccinated with Vaxzevria. Doctors should ask about this during the preliminary talk about the vaccination.
CLS is considered a rare disease. It is characterized by a misdirected inflammatory reaction and a dysfunction of the blood and lymph vessels. In the specific case, this means that the mechanisms of vasodilation are disrupted and the blood vessels become permeable for the duration of the CLS episode.
As a direct consequence, the blood pressure of the affected person drops rapidly and there is an influx of fluid into the tissues. This results in rapid weight gain as the swelling of the arms and legs progresses. This in turn leads to a steady thickening of the blood (hemoconcentration), which can possibly lead to organ failure or shock.
The PEI points out that in rare cases systemic CLS can also be triggered by Covid-19 infections.
Also compatible with allergies
According to the current state of knowledge, the vaccine is also suitable for allergy sufferers. Allergy sufferers should inform their vaccinating doctor about known allergies before vaccination. In the event of an allergic reaction, the doctor can quickly take countermeasures.
In addition, after the vaccination, you should remain in the practice or in the vaccination center for at least 15 minutes for medical supervision
Vaccination during pregnancy
There is no experience of using the vaccine during pregnancy. According to a preliminary assessment by the EMA, no harmful effects on the unborn child are to be expected from vaccination with Vaxzevria (AZD1222).
However, this assessment is based on preliminary studies in animal models. Reliable data on the effects and side effects in pregnancy are not yet available for Vaxzevria (AZD1222).
The decision as to whether a vaccination during pregnancy makes sense should be clarified in close consultation with your doctor. He can best assess the benefits and risks for you.
Vaccination in case of illness
According to the EMA, you can get vaccinated if you have mild cold symptoms. In the event of a more severe illness, however, you should postpone an upcoming vaccination.
Vaccination and anticoagulants
People who take preventive anticoagulants should make the doctor aware of them in advance. The general precautionary measures then apply: In the case of anticoagulation therapy, the vaccine must be administered with particular care.
Vaccination with immunodeficiency
How well Vaxzevria (AZD1222) works in patients with immunodeficiency or drug-suppressed defenses (immunosuppression) and how well they tolerate it is currently unclear. No studies in this regard have taken place so far. According to the EMA, however, it is possible that the effect of the vaccination could be less in patients with a weak immune system.
Storage and shelf life
In contrast to the tried and tested Comirnaty vaccines from BioNTech / Pfizer and the vaccine from Moderna, Vaxzevria (AZD1222) can be stored in the refrigerator for a longer period of time.
The maximum storage time specified by the manufacturer in the unopened state is around six months. Vaxzevria (AZD1222) is supplied in jars with 8 or 10 doses each.