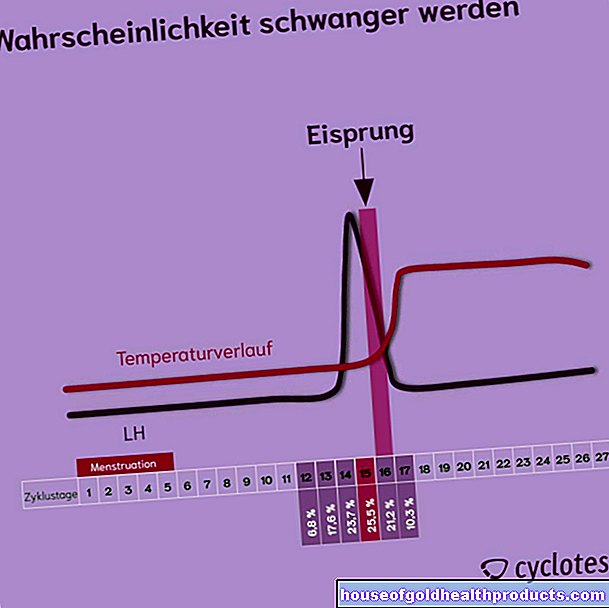
Corona vaccine: is the first approval coming soon?
Lisa Weidner studied German and sociology and completed several journalistic internships. She is a volunteer at Hubert Burda Media Verlag and writes for the "Meine Familie und Ich" magazine and on nutrition and health topics.
More about the experts All content is checked by medical journalists.
The world is waiting for a corona vaccine. The German manufacturer Biontech is now presenting interim results of a large study. A US virologist speaks of the best news since the beginning of the pandemic, experts expect approval soon.
An effective corona vaccine from Europe and the USA is within reach: The Mainz-based company Biontech and the pharmaceutical company Pfizer were the first western manufacturers to publish promising results of a study on Monday that is crucial for approval. Accordingly, the vaccine offers more than 90 percent protection against the disease Covid-19. Serious side effects have not yet been registered, it is said.
Apply for admission next week?
Biontech and Pfizer are expected to apply for approval from the US Food and Drug Administration (FDA) starting next week.
Federal Health Minister Jens Spahn (CDU) said that he was assuming a parallel application for approval from the European Medicines Agency (EMA). As German health minister, he wanted to ensure that a vaccine from a German company "is not first available in other countries".
On the part of Biontechs it was said that the available cans should be distributed "fairly". It will not "one country get it all".
To distribute a vaccine within Germany, leading health advisors to the German Ethics Commission, the Standing Vaccination Commission and the Robert Koch Institute presented a paper on Monday that recommends prioritization according to risk groups.
First wave of vaccinations at the end of 2020?
In the event of approval, Clemens Wendtner from the Munich Clinic Schwabing is hoping for a quick vaccination start: "If this step is taken, a wave of vaccinations could indeed start by the end of 2020." The presented results are a "silver lining on the otherwise gloomy horizon". The virologist Florian Krammer from the Icahn School of Medicine in New York called the announcement the "best news" for him since the beginning of the pandemic.
"Speed of Light" project
Biontech had been developing the BNT162b2 vaccine in the "Lightspeed" project since mid-January. The phase 3 study, which is crucial for approval, started in various countries at the end of July. By Monday, more than 43,500 people had received at least one of the two vaccinations, which are given every three weeks. According to the manufacturer, vaccination protection is achieved one week after the second injection.
90 percent protection possible
In the study, a total of 94 Covid 19 cases were confirmed by Sunday - 90 percent of them in the control group that had received the placebo preparation. The very high effectiveness that emerges makes it possible to apply for approval in advance.
According to Biontech and Pfizer, the results are only finally evaluated when a total of 164 cases have been reached. In addition, it is checked to what extent the vaccination not only protects against Covid-19, but also against severe courses of the disease. Overall, both the protective effect and any side effects should be observed over a period of two years.
Data on the duration of action and certain age groups are still lacking
The infectiologist Gerd Fätkenheuer from the Cologne University Hospital spoke of "great and promising data". "I think this will have a decisive impact on how we deal with the pandemic, and I hope that large quantities of the vaccine will be available quickly."
However, experts also indicated that the data initially only came from a press release and not from a scientific publication. For example, there was a lack of information on the protective effect in certain age groups and on how long vaccination protection lasts.
First RNA vaccine worldwide
The Biontech preparation is a so-called RNA vaccine, which is based on a completely new mechanism. It contains genetic information about the pathogen, from which the body produces a virus protein - in this case the surface protein that the virus uses to penetrate cells. The aim of vaccination is to stimulate the body to produce antibodies against this protein in order to intercept the viruses before they enter the cells and multiply.
50 million vaccine doses still in 2020
One advantage of RNA vaccines is that they can be produced much faster than conventional vaccines. Biontech and Pfizer expect to provide up to 50 million vaccine doses worldwide this year, and up to 1.3 billion doses in the coming year.
The EU Commission has been negotiating for some time with Biontech / Pfizer about a framework agreement for the delivery of the vaccine to all EU countries. Commission President Ursula von der Leyen wrote on Monday afternoon on Twitter that a contract for up to 300 million vaccine doses will soon be signed. So far, the Commission has signed framework agreements with the pharmaceutical companies Johnson & Johnson, Astrazeneca and Sanofi-GSK.
An accelerated approval process applies to corona vaccines due to their particular urgency. At the EMA, pharmaceutical manufacturers can submit individual parts on the quality, harmlessness and effectiveness of a preparation even before the complete application for approval. In addition to Biontech, the British-Swedish company Astrazeneca has started such a rolling review process for its vaccine candidate. Astrazeneca has not yet released any Phase III data. Nothing can be said about the schedule, a spokeswoman said on Monday.
International: First vaccines already approved
Countries such as Russia, China and recently Bahrain have already released vaccines with restrictions and are already vaccinating parts of the population with them. But how well these vaccinations actually protect and what side effects they can have is currently largely open.
The publication of the interim results also gave the stock exchanges in Europe a strong boost on Monday: The Dax conquered the 13,000-point mark for the first time since mid-October and rose by 4.6 percent to 13,051.63 points by midday. The leading Eurozone index EuroStoxx 50 gained 4.9 percent to 3362.09 points. In pre-market US trading, the papers of the US pharmaceutical company Pfizer initially rose by around 9 percent, while the Biontech papers shot up by around 16 percent. Also (still) President Donald Trump reacted enthusiastically. "Such great news!" He tweeted in capital letters. (lw / dpa)
Tags: unfulfilled wish to have children therapies prevention






.jpg)



.jpg)