The hunt for the straw
Dr. Andrea Bannert has been with since 2013. The doctor of biology and medicine editor initially carried out research in microbiology and is the team's expert on the tiny things: bacteria, viruses, molecules and genes. She also works as a freelancer for Bayerischer Rundfunk and various science magazines and writes fantasy novels and children's stories.
More about the experts All content is checked by medical journalists.Hannah has to die. Although there is a drug for her child dementia. In such a case, can the pharmaceutical company really refuse to release the drug - just because it has not yet been approved?
Hannah is sitting at the crafting table and gluing wings to a butterfly. The nine-year-old girl with the brown, slightly curly hair loves to do handicrafts. But soon she will no longer be able to do that. Because Hannah suffers from a rare disease: neuronal ceroid lipofuscinosis type 2, or NCL2 for short. The writing Hannah scratches on the butterfly can hardly be deciphered. Hannah can already no longer read or write properly - she is suffering from dementia. As the disease progresses, it will also unlearn to walk and speak - and it will go blind. The young patients with NCL2 also often have hallucinations and epileptic seizures. Most die in their teens.
For Hanna's parents, the diagnosis in February came as a great shock. “First I had to go outside to cry,” Hannah's mom Stefanie Vogel told But then the family found out about a drug that might help Hannah. The catch: It is still in the clinical test phase and will be approved in two to three years at the earliest. It's too late for Hannah. "We will do everything we can to ensure that Hannah gets the medication as soon as possible," says the mother. But is that even possible?
Exceptions allowed
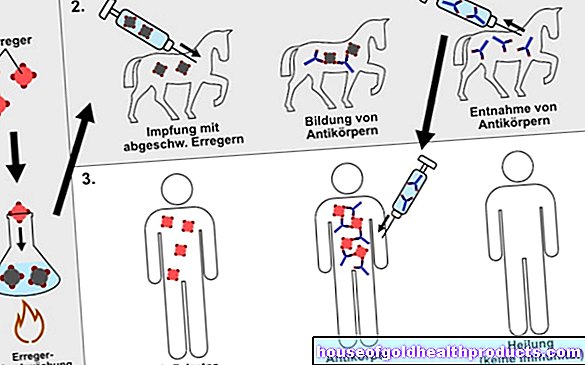
Before a new drug comes onto the market, clinical studies must determine whether it works and whether it is tolerated. In Germany, however, there is the possibility of a so-called individual attempt at healing. If someone is terminally ill like Hannah, the manufacturer is allowed to release the drug for this patient before approval, writes the Association of Research-Based Drug Manufacturers (vfa). The prerequisite is that all other therapy options have been exhausted and that a doctor is found to take over the treatment.
Little Hannah would have met all the criteria for an individual attempt at healing. Children with NCL2 lack a specific enzyme, tripeptidyl peptidase 1 (TPP 1). This is, so to speak, the cell's own garbage disposal. Without this, waxy substances accumulate in the tissues. The sensitive nerve cells gradually die off as a result.
Replaced cell garbage disposal
"It makes sense to replace the missing TPP 1," says Professor Thorsten Marquardt from the University of Münster. The metabolism expert has often treated patients with drugs that have not yet been approved. He would also oversee Hannah's therapy.
Cerliponase Alfa or BMN 190 is the name of the drug from the US company Biomarin that could free Hannah's nerve cells from rubbish. So that the artificially produced enzymes arrive directly on the spot, a small tube is put through a mini hole in the skull directly into the brain of the children, which is connected to a small storage tank. This then continuously releases the replacement enzyme to the nerve cells in the brain.
Denied enzyme replacement therapy
An international clinical test with 24 NCL2 children has been running for one and a half years. According to the manufacturer, none of the small test subjects has worsened since they started taking the drug. Some even report an improvement.
But Biomarin doesn't want to release the life-saving drug to treat Hannah. According to vfa, the decision then actually lies with the research-based pharmaceutical company. It has to be weighed up whether there is a chance or a risk for the patient in a specific case.
Fight against a pharmaceutical giant
In fact, when asked by, Biomarin writes that the drug's safety and effectiveness have not yet been sufficiently proven at this point in time. But the argument lags: “There is no alternative for Hannah. In one to two years, her brain will be so destroyed that she needs care, ”says Stefanie Vogel. In addition, the pharmaceutical company refers to the “ethical obligation” that one has towards all patients with NCL2, “who are also waiting with the greatest urgency for new therapeutic options.
There are therefore fears that approval of the drug could be delayed if it were released to patients outside the study. The risk is definitely there, namely if something goes wrong in the individual attempt at healing. Then, for example, participants in the study could drop out or the licensing authority could make a negative judgment, explains Dr. Rolf Hömke from vfa told about the problem. In that case, other patients would actually have to wait longer for a drug. Most of all, Biomarin would lose a lot of money.
Hannah and her family don't want to accept that. You started a petition on change.org that was signed by more than 200,000 people in no time. It remains to be seen whether they can change the mind of the pharmaceutical giant. Hannah has also applied for a second clinical trial starting in December. Nobody knows how far the disease will have progressed by this point in time.
Link to the collection of signatures "Hope for Hannah":https://www.change.org/hannah
Tags: healthy feet diet vaccinations