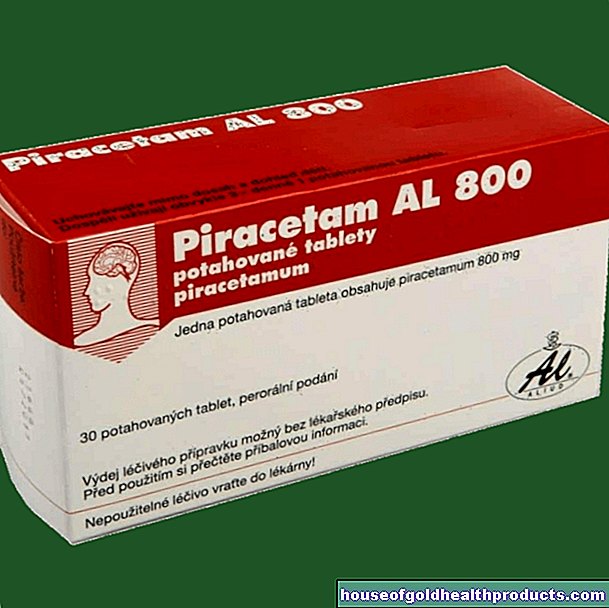
Remdesivir: around 2000 euros per patient
Lisa Vogel studied departmental journalism with a focus on medicine and biosciences at Ansbach University and deepened her journalistic knowledge in the master's degree in multimedia information and communication. This was followed by a traineeship in the editorial team. Since September 2020 she has been writing as a freelance journalist for
More posts by Lisa Vogel All content is checked by medical journalists.The drug Remdesivir can reduce the hospital days of seriously ill Covid-19 patients by an average of four days. Now the manufacturer, the US biotech company Gilead Sciences, has set the price for the drug.
Remdesivir is currently only intended for use in hospitals. A five-day treatment with the drug would cost 2340 dollars (about 2000 euros) per patient when ordered by the US government, wrote Gilead boss Daniel O "Day in an open letter on Monday. Private health insurers in the US should be around a third more Pay to offset appropriate set discounts.
The price now set is below the $ 5080 recommended by the US Research Group on Drug Prices (ICER). Nevertheless, it can be assumed that the Gilead company will use the funds to generate revenues in the billions over the next few years.
Treatment aims to reduce hospital costs
On average, the drug should reduce hospital costs by $ 12,000 per patient, quoted the news platform ntv O "Day. Gilead calculates the savings on the basis of data that each day of hospitalization costs $ 3,000 and that patients who take remdesivir four days earlier are discharged than those receiving standard care.
Corresponding prices will also apply in other developing countries, including Germany. There will be no higher prices for private health insurance companies either. There will be generic, lower-priced versions of the drug for countries in need. According to O "Day, everyone would have access to the active ingredient.
In fact, according to a report by Zeit-online, the USA bought up almost all of the expected production of the product by the end of September. All other countries will therefore only be able to expect deliveries from autumn onwards.
Remdesivir also recommended in Europe
Just a few days ago, the European Medicines Agency (EMA) recommended approval for the drug with the trade name Veklury, subject to certain conditions, in Europe as well. An international study with over 1000 participants showed at the end of April that Remdesivir can reduce the time to recovery in Covid-19 patients by an average of four days - from 15 to 11 days. The mortality decreased slightly in the study, but this was not statistically significant.
The USA had already issued an exemption for the limited use of the active ingredient in hospitals at the beginning of May. In Germany, too, the drug was already available within a compassionate use program and is being tested in clinical studies.
Remdesivir was originally developed to treat Ebola, but it was not effective enough. So far, it has not been unrestrictedly approved as a drug in any country in the world. So far there has been no vaccination against the novel coronavirus and no reliable, approved drug therapy. (lv / dpa / ntv)
Tags: first aid menshealth travel medicine